Tungsten hexafluoride is also called tungsten (VI) fluoride with CAS No. is 7783-82-6 and molecular formula of WF6.
Tungsten hexafluoride is a colorless diamagnetic gas at ambient pressure and temperatures above 17 °C. The WF6 molecule is octahedral. And it condenses into a pale yellow liquid between 2.3 and 17°C with the density of 3.44g/cm3 at 15°C. At 2.3°C, it freezes into a white solid having a cubic crystalline structure with calculated density 3.99g/cm3.
Whereas WF6 gas is one of the heaviest gases, with the density exceeding that of the heaviest elemental gas radon (9.73g/L), the density of WF6 in the liquid and solid state is rather moderate.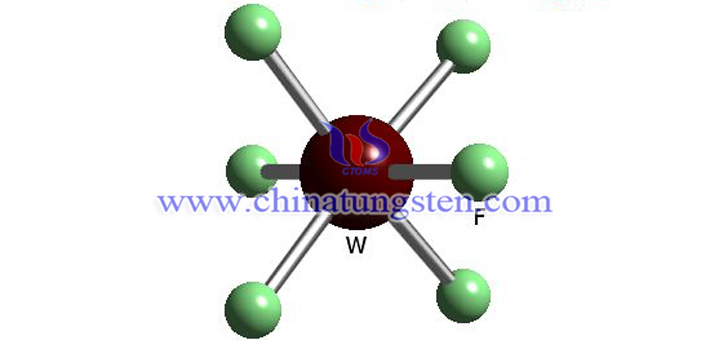
Properties
1. Decomposed in water to form tungsten trioxide and hydrogen fluoride (HF).
2. Soluble in benzene and other organic solvents with special color.
3. A strong fluorinating agent that allows many metals to be fluorinated at room temperature except stainless steel, nickel, etc.
4. React with ionic type halides to form coordination compounds.
5. React with almost all metals except gold and platinum (Pt).
6. Reduced by hydrogen to tungsten under high temperature.
7. Form double salt with alkali fluoride.
8. Absorbed by alkali or ammonium hydroxide.
More details, please visit:
http://tungstate.net/English/hexafluoro-tungsten.html




