WO3, or tungsten trioxide, is an important electrochromic material, which is more and more widely used in the production of electrochromic devices, and therefore it is applied in smart windows. So, what is the structure of the tungsten trioxide raw material? What are the basic physicochemical properties of tungsten trioxide?
More details, please visit:
http://www.tungsten-powder.com/tungsten-oxide.html
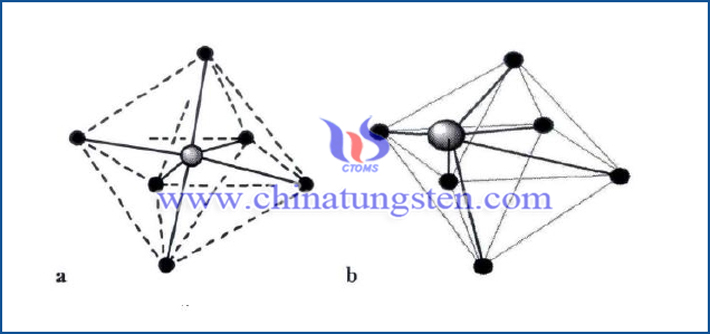
The ideal WO3 crystal is a perovskite structure composed of a [WO6] regular octahedron with W atoms located at the center and O atoms at the six vertices of the regular octahedron, as a shown above. However, the actual WO3 does not strictly meet the stoichiometric ratio, and the octahedral unit will undergo different degrees of tilt or rotation. The tungsten atom deviates from the octahedral body center, and the crystal structure is a distorted perovskite structure, as b shown above. In addition, tungsten trioxide is a yellow powder, which is insoluble in water and acid, slightly soluble in HF, soluble in hot alkali and ammonia. Tungsten trioxide is a transition metal oxide with an optical band gap of about 2.9eV at room temperature. So, tungsten trioxide is also known as a wide band gap semiconductor.




