Crystalline tungsten trioxide can be used to prepare electrochromic film. For the crystalline WO3, experts say that, ideally, it can be considered as a deformed perovskite crystal structure (as shown below). The chemical bond is the ionic bond between W6+ and O2-, but it also has an obvious covalent component.
More details, please visit:
http://www.tungsten-powder.com/tungsten-oxide.html
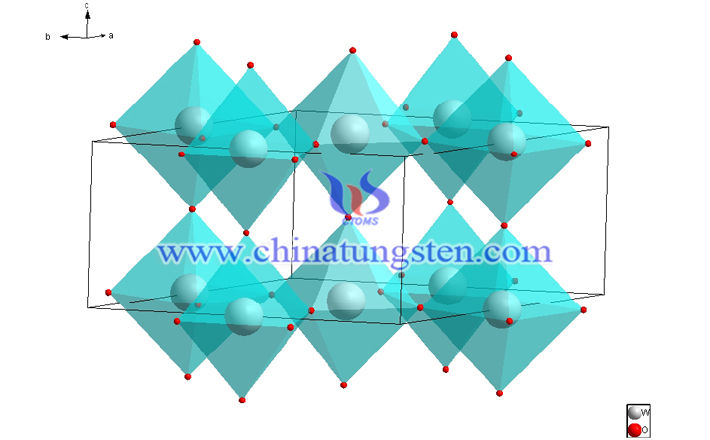
Experts also explained that this distorted perovskite structure consists of [WO6] octahedrons, which are arranged in a co-angled manner. The W atoms are located in the center of the octahedron, and each oxygen atom is used as bridge oxygen for the two [WO6] regular octahedrons. Like other metal oxides, the WO3 phase changes with temperature as follows:
Monoclinic II (ε-WO3, <-43°C) → triclinic (δ-WO3, -43°C to 17°C) → monoclinic I (γ-WO3, 17°C to 330°C) → orthorhombic (β-WO3 , 330°C to 740°C) → tetragonal (α-WO3, > 740°C).




